|
|
Vol. 4 Iss. 3 The Chemical Educator © 1999 Springer-Verlag New York, Inc. |
ISSN 1430-4171 |
|
|
Vol. 4 Iss. 3 The Chemical Educator © 1999 Springer-Verlag New York, Inc. |
ISSN 1430-4171 |
The ElemenTree is available in two versions: 1. A transparent Vivak plastic periodic tree in pink, green, and black, 10-1/2 in. high, 8 in. at widest near base, US $31.00. 2. A black-and-white Mini-ElemenTree, which fits into the pocket of a 3-ring loose-leaf binder, US $18.00. Both kits include a 24-page instruction manual. Prices do not include shipping and handling or sales tax. Order as Catalog No. M2-84-0124 from Carolina Biological Supply Co., 2700 York Road, Burlington, NC 27215, Telephone: (800) 334-5551; FAX: (800) 222-7112; e-mail: dthomas@carolina.com or fcherry@carolina.com . http://www.carolina.com .
Readers of two recent articles by Eric Scerri
on the periodic table (American Scientist, Nov.- Dec. 1997, pp
546 - 553 and Scientific American, Sept. 1998, pp. 78 - 83)
may have seen colored pictures of a 3-dimensional periodic table and
wondered if it is commercially available. The answer is a resounding
yes.
According to the late American astronomer
Harlow Shapley, the periodic table "is probably the most compact and
meaningful compilation of knowledge that man has yet devised." Since
Dmitrii Ivanovich Mendeleev first published it in 1869, more than 700
different graphical representations of this cornerstone of modern
inorganic chemistry have been published (e.g., see Edward G. Mazurs,
Graphic Representations of the Periodic System During One Hundred
Years; University of Alabama Press, 1974, for a compilation).
Each has advantages and disadvantages, and the quest for an "ideal"
or "perfect" periodic table continues unabated.

Since he first saw an article featuring an adaptation of the 1925 Courtine model in the November 6, 1946 issue of Popular Science magazine, Fernando Dufour, chemistry professor at the Collège Ahuntsic, Montréal, Québec, Canada (now emeritus), better known to his friends and aficionados as "Nando," has been fascinated and obsessed by the idea of developing a 3-D color model table of his own.
He has described the genesis of his lifelong passionate preoccupation: "When I discovered the periodic table, I was awed to the ultimate heaven in thinking that this was knowledge so infinite it would unravel all the mysteries of nature - the blueprint of the universe itself. It was Archimedes' grain of sand - 'To understand a grain of sand is to understand the universe.'" Since then, for more than half a century, this septuagenarian, who humorously refers to himself as "a 74-year-old kid," has spent all his time developing version after version of a three-dimensional periodic table (first using cardboard and Styrofoam, and now plastic) designed for teachers, students, or for classroom use via an overhead projector, which makes one model sufficient for an entire class of students. In 1979 he received his M.Sc. degree from the Université Concordia in Montréal with the thesis topic, "An attempt to unravel atomic structure with a three dimensional model of the periodic table." In his opinion, "A third dimension [for the periodic table] is not an option but a necessity."
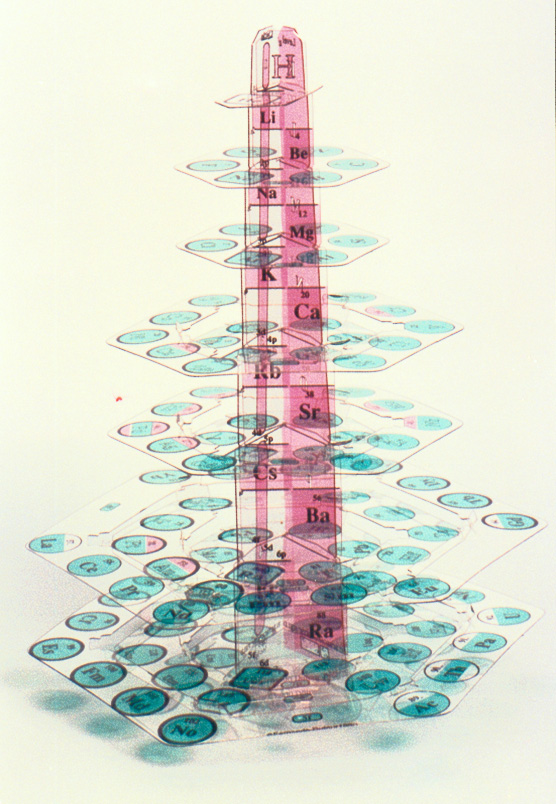
Figure 2. The ElemenTree
Easily assembled from 17 dismountable components, with s-block elements, the alkali and alkaline earth metals (pink), forming the trunk and the p-, d-, and f-block elements (green) arranged concentrically as hexagonal branches, this latest version of Dufour's model resembles a stylized Christmas tree (sapin périodique). Used in the first Chemical Heritage Foundation - Woodrow Wilson Summer Institute on the History of Chemistry at Princeton University (July 5-31, 1992) by Susana S. Suarez, it was displayed at the Annual Chemistry Congress held in Manchester, England and has been featured in numerous newspaper and magazine articles.
As students assemble the tree, they effortlessly apply the Aufbauprinzip and can simultaneously visualize and trace trends, similarities, and differences in a variety of fundamental concepts such as the continuity and periodicity of an unbroken sequence of elements from hydrogen (atomic no. 1) at the top to ununoctium (atomic no. 118) at the base, Pauli's exclusion principle, Hund's rule, vertical and horizontal symmetry (groups and periods), quantum numbers, valence, order of orbital energies, types of orbitals involved, atomic radius, ionic or covalent bonding, sigma-pi bonding, metallic or nonmetallic character, ionization potential, electronegativity, and electron affinity. Many chemical or physical properties of the elements that are discerned only with difficulty or not at all with the traditional two-dimensional periodic table are readily perceived and correlated by use of the 3-D periodic tree; the accompanying instruction manual describes how, with both its vertical and horizontal axes, the ElemenTree expands visual access from the three periodic features of the two-dimensional table to 42 features. By removing the central support, the preassembled parts fit into a book-size (8-5/8 in. x 9-1/2 in.) folder, ready to be "recycled" from one classroom to another or to be stored on a bookshelf.
This useful educational and research tool suitable for high school through college and university level courses in chemistry and related fields has been sold in 26 countries, 46 of the United States, and 10 Canadian provinces. To the best of my knowledge there is no other product like it on the market. Indeed, Sherlock Holmes may have anticipated Dufour's pedagogically ingenious, user-friendly, hands-on interactive 3-D periodic table when he said to Dr. Watson, "ElemenTree."